Similar to myelopoiesis, the process of lymphopoiesis involves the progression of hematopoietic stem cells through several stages in order to generate lymphoid precusors. T cells, B cells, plasma cells, and natural killer cells can all be derived from these precursors. T cells and B cells in particular undergo stringent methods of selection in an effort to create cells that can effectively combat foreign pathogens yet remain non-reactive to self.
Explore our interactive graphics below by clicking on each cell type to bring up a list of markers expressed at that stage of development.
|
|
|



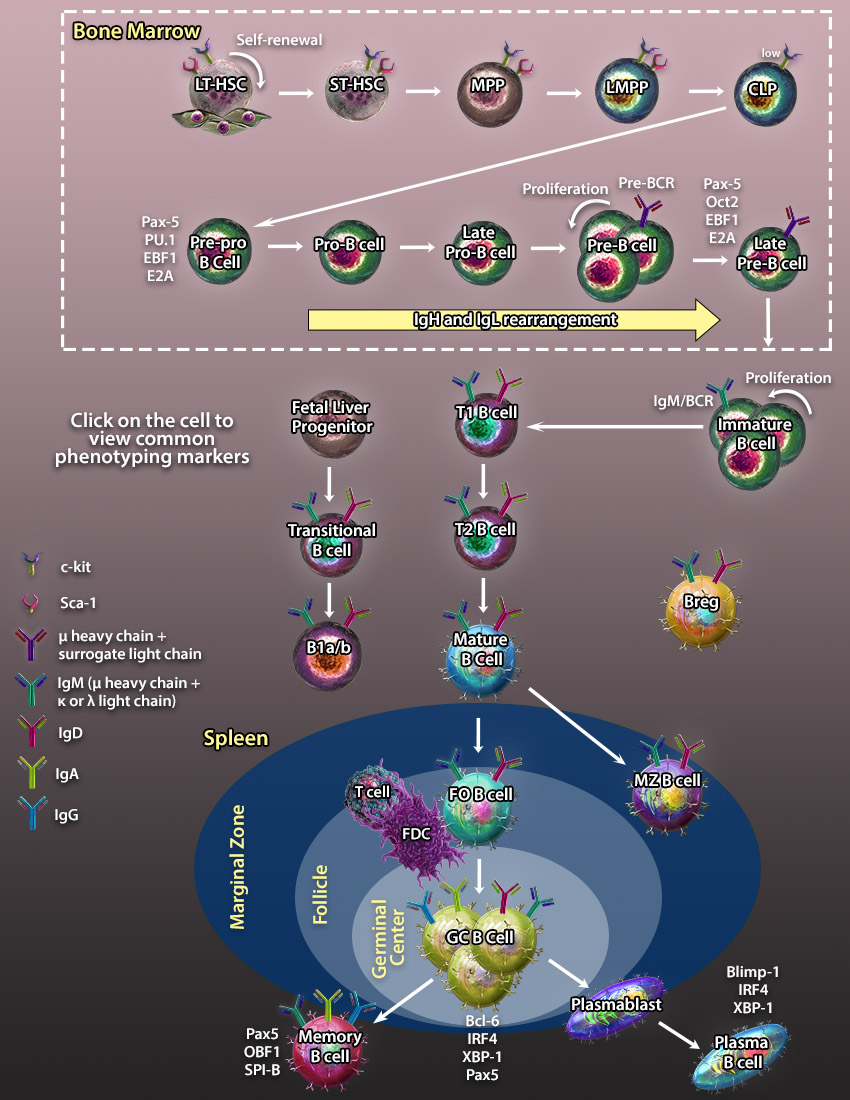
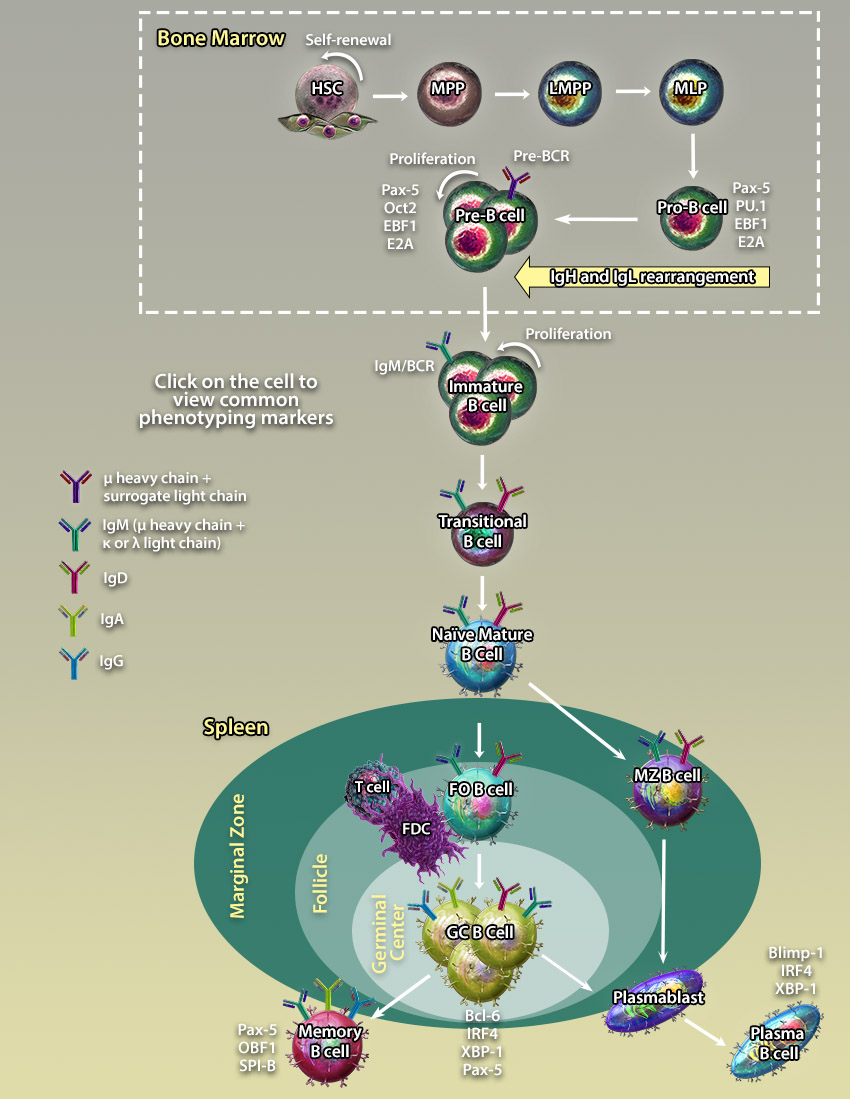

 LT-HSCs are pluripotent cells which give rise to all blood cell populations of lymphoid, myeloid, and erythroid lineages and persist throughout the entire lifespan. They have the potential to self-renew sustaining the stem cell pool or differentiate into ST-HSCs and other multi-, oligo-, and unipotent progenitors which give rise to terminally differentiated cells.
LT-HSCs are pluripotent cells which give rise to all blood cell populations of lymphoid, myeloid, and erythroid lineages and persist throughout the entire lifespan. They have the potential to self-renew sustaining the stem cell pool or differentiate into ST-HSCs and other multi-, oligo-, and unipotent progenitors which give rise to terminally differentiated cells. MPPs are multipotent progenitors derived from ST-HSCs. They can give rise to another multipotent progenitor, LMPPs, or oligopotent and unipotent progenitors which give rise to terminally differentiated cells.
MPPs are multipotent progenitors derived from ST-HSCs. They can give rise to another multipotent progenitor, LMPPs, or oligopotent and unipotent progenitors which give rise to terminally differentiated cells. LMPPs are responsible for the generation of lymphocytes and pDCs through subsequent differentiation steps including CLPs and pre-pDCs respectively. LMPPs were originally thought to be a homogeneous population of precursors containing multipotent cells with the equal potential to differentiate into several lymphoid lineages. However, a recent study has demonstrated that LMPP is a heterogeneous population of cells comprising unipotent clones with a non-overlapping lineage differentiation potential(21).
LMPPs are responsible for the generation of lymphocytes and pDCs through subsequent differentiation steps including CLPs and pre-pDCs respectively. LMPPs were originally thought to be a homogeneous population of precursors containing multipotent cells with the equal potential to differentiate into several lymphoid lineages. However, a recent study has demonstrated that LMPP is a heterogeneous population of cells comprising unipotent clones with a non-overlapping lineage differentiation potential(21). Pre-pro B cells are differentiated from CLPs and are the earliest B cell progenitor identified in the bone marrow.
Pre-pro B cells are differentiated from CLPs and are the earliest B cell progenitor identified in the bone marrow.
 Late Pro-B cells undergo rearrangement of the V segment of the µ-heavy chain. Upon V segment rearrangement, it joins the DJ segments to form a mature µ-heavy chain. Late Pro-B cells which have successfully rearranged a heavy chain that is capable of pairing with surrogate light chains (VpreB and λ5) to form a pre-BCR (B cell receptor) are allowed to progress to the next stage.
Late Pro-B cells undergo rearrangement of the V segment of the µ-heavy chain. Upon V segment rearrangement, it joins the DJ segments to form a mature µ-heavy chain. Late Pro-B cells which have successfully rearranged a heavy chain that is capable of pairing with surrogate light chains (VpreB and λ5) to form a pre-BCR (B cell receptor) are allowed to progress to the next stage. Upon upregulation of the pre-BCR on the surface of Pre-B cells, the expression of RAG1/2 recombinases is downregulated and Pre-B cells undergo several divisions before they enter the Late Pre-B cell stage.
Upon upregulation of the pre-BCR on the surface of Pre-B cells, the expression of RAG1/2 recombinases is downregulated and Pre-B cells undergo several divisions before they enter the Late Pre-B cell stage.
 After a second wave of RAG1 and RAG2 recombinase upregulation, Pre-B cells undergo V and J segment rearrangement and diversification to form λ and κ light chains. Upon successful pairing of light chain with the µ-heavy chain, the B cell receptor associates with Ig-α and Ig-β molecules to form a fully functioning receptor.
After a second wave of RAG1 and RAG2 recombinase upregulation, Pre-B cells undergo V and J segment rearrangement and diversification to form λ and κ light chains. Upon successful pairing of light chain with the µ-heavy chain, the B cell receptor associates with Ig-α and Ig-β molecules to form a fully functioning receptor. T1 B cells differentiate from immature B cells which have successfully passed negative selection. Transitional B cell maturation occurs primarily in the spleen, with T1 B cells present in the red pulp. Upon encountering antigen, T1 B cells differentiate to T2 B cells which are able to give rise to follicular, marginal zone, germinal center, memory, and plasma B cells.
T1 B cells differentiate from immature B cells which have successfully passed negative selection. Transitional B cell maturation occurs primarily in the spleen, with T1 B cells present in the red pulp. Upon encountering antigen, T1 B cells differentiate to T2 B cells which are able to give rise to follicular, marginal zone, germinal center, memory, and plasma B cells. Mature B lymphocytes develop sequentially from transitional type 1 (T1) and type 2 (T2) precursors in the spleen. They can be distinguished from their progenitors by expression of both IgM and IgD, as well as a lack of Immature B cell marker CD24. Mature B cells can further differentiate into germinal center and marginal zone B cells.
Mature B lymphocytes develop sequentially from transitional type 1 (T1) and type 2 (T2) precursors in the spleen. They can be distinguished from their progenitors by expression of both IgM and IgD, as well as a lack of Immature B cell marker CD24. Mature B cells can further differentiate into germinal center and marginal zone B cells. Follicular B cells can be found within structures called follicles (which contain germinal centers) in secondary and tertiary lymphoid organs such as the spleen, Peyer's patches, and lymph nodes. Unlike marginal zone B cells, Follicular B cells circulate throughout the body. They are capable of becoming either Memory B cells or Plasma cells. In most instances, these cells require T cells for full activation.
Follicular B cells can be found within structures called follicles (which contain germinal centers) in secondary and tertiary lymphoid organs such as the spleen, Peyer's patches, and lymph nodes. Unlike marginal zone B cells, Follicular B cells circulate throughout the body. They are capable of becoming either Memory B cells or Plasma cells. In most instances, these cells require T cells for full activation. Upon antigen encounter and interaction with T follicular cells, B cells cluster into germinal centers within follicles. B cell maturation in GCs is associated with somatic hypermutation of antibody V regions allowing the cells to generate antibodies with high avidity BCRs. Antigen selected GC B cells leave the germinal center to differentiate further into memory or plasma B cells.
Upon antigen encounter and interaction with T follicular cells, B cells cluster into germinal centers within follicles. B cell maturation in GCs is associated with somatic hypermutation of antibody V regions allowing the cells to generate antibodies with high avidity BCRs. Antigen selected GC B cells leave the germinal center to differentiate further into memory or plasma B cells. Marginal Zone (MZ) B cells are non-circulating B cells which can be identified in the marginal zone of lymphoid organs such as the spleen. They typically have a lower threshold of activation compared to follicular B cells and can develop into plasma cells with the help of T cells.
Marginal Zone (MZ) B cells are non-circulating B cells which can be identified in the marginal zone of lymphoid organs such as the spleen. They typically have a lower threshold of activation compared to follicular B cells and can develop into plasma cells with the help of T cells. Memory B cells are found predominantly in the marginal zone of the spleen, the sub-capsular sinus of the lymph nodes, and under the intestinal epithelium in Peyer's patches and crypt epithelium of the tonsils. Memory B cells develop after infection and retain memory of the antigen allowing for a more rapid immune response upon reintroduction of the antigen.
Memory B cells are found predominantly in the marginal zone of the spleen, the sub-capsular sinus of the lymph nodes, and under the intestinal epithelium in Peyer's patches and crypt epithelium of the tonsils. Memory B cells develop after infection and retain memory of the antigen allowing for a more rapid immune response upon reintroduction of the antigen. Plasma B cells are terminally differentiated B cells which circulate throughout the body and produce large volumes of antibodies. They can be identified by high levels of expression of CD27 and CD138.
Plasma B cells are terminally differentiated B cells which circulate throughout the body and produce large volumes of antibodies. They can be identified by high levels of expression of CD27 and CD138. Plasmablasts are located in the peripheral immune organs where they undergo rapid proliferation to produce a large pool of terminally differentiated plasma cells. They can be distinguished from plasma cells by low production antibodies, ability to proliferation, and a shorter lifespan.
Plasmablasts are located in the peripheral immune organs where they undergo rapid proliferation to produce a large pool of terminally differentiated plasma cells. They can be distinguished from plasma cells by low production antibodies, ability to proliferation, and a shorter lifespan. B1 cells are derived from fetal progenitors and are localized mainly in the peritoneal cavity and gut-associated lymphoid tissues. B1-a and B1-b cells can be distinguished by the expression of CD5. The B1 BCR is less diverse than that of B2 cells as it is rearranged from a limited number of Ig gene segments and lacks nucleotide editing. B1 cells predominantly secrete IgM and undergo very little somatic hypermutation.
B1 cells are derived from fetal progenitors and are localized mainly in the peritoneal cavity and gut-associated lymphoid tissues. B1-a and B1-b cells can be distinguished by the expression of CD5. The B1 BCR is less diverse than that of B2 cells as it is rearranged from a limited number of Ig gene segments and lacks nucleotide editing. B1 cells predominantly secrete IgM and undergo very little somatic hypermutation. Regulatory B cells represent a small fraction of B cells with immunosuppressive capacities. These cells can be phenotypically challenging to identify, as they bear many common markers found on other B cell types.
Regulatory B cells represent a small fraction of B cells with immunosuppressive capacities. These cells can be phenotypically challenging to identify, as they bear many common markers found on other B cell types.
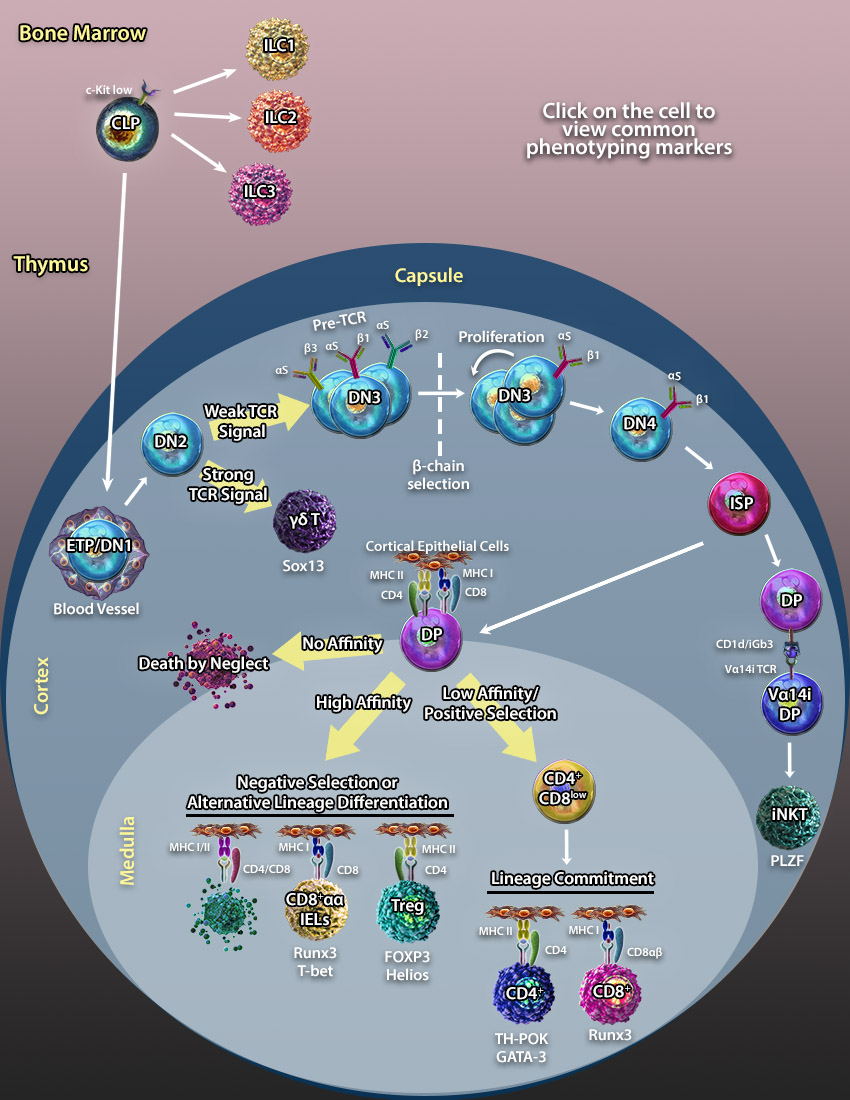
 Like T cells, ILCs are derived from CLPs; however, they do not respond in an antigen-specific manner. Group 1 ILCs include Natural Killer (NK) cells and produce type 1 cytokines including IFN-γ and TNF-α. They play a critical role in protecting the host against pathogens and tumor cells.
Like T cells, ILCs are derived from CLPs; however, they do not respond in an antigen-specific manner. Group 1 ILCs include Natural Killer (NK) cells and produce type 1 cytokines including IFN-γ and TNF-α. They play a critical role in protecting the host against pathogens and tumor cells. Like T cells, ILCs are derived from CLPs; however, they do not respond in an antigen-specific manner. Group 2 ILCs produce cytokines normally associated with Th2 cells including IL-5 and IL-13. They play a key role in response to helminth infection, allergic lung inflammation, and are involved in wound healing.
Like T cells, ILCs are derived from CLPs; however, they do not respond in an antigen-specific manner. Group 2 ILCs produce cytokines normally associated with Th2 cells including IL-5 and IL-13. They play a key role in response to helminth infection, allergic lung inflammation, and are involved in wound healing. Like T cells, ILCs are derived from MLPs; however, they do not respond in an antigen-specific manner. Group 3 ILCs were originally discovered as cells that express NKp46, but do not resemble normal NK cells. Instead, they express RORγt and produce IL-17 and IL-22. ILC3s primarily reside in mucosal tissues where they help maintain intestinal homeostasis. Lymphoid tissue inducer cells (LTi) are considered subset of group 3 ILCs that are involved in lymphoid organ formation during embryogenesis. The exact relationship between LTis and other ILC3s is still being investigated.
Like T cells, ILCs are derived from MLPs; however, they do not respond in an antigen-specific manner. Group 3 ILCs were originally discovered as cells that express NKp46, but do not resemble normal NK cells. Instead, they express RORγt and produce IL-17 and IL-22. ILC3s primarily reside in mucosal tissues where they help maintain intestinal homeostasis. Lymphoid tissue inducer cells (LTi) are considered subset of group 3 ILCs that are involved in lymphoid organ formation during embryogenesis. The exact relationship between LTis and other ILC3s is still being investigated. ETP/DN1s are one of the first T cell progenitor populations that have been identified in the thymus. These progenitors are named double negative as they do not express the CD4 or CD8 co-receptors found on mature T cells. Additionally, they do not express the CD3/TCR complex.
ETP/DN1s are one of the first T cell progenitor populations that have been identified in the thymus. These progenitors are named double negative as they do not express the CD4 or CD8 co-receptors found on mature T cells. Additionally, they do not express the CD3/TCR complex. DN2 thymocytes are differentiated from DN1 progenitors. These progenitors were named double negative as they do not express the CD4 or CD8 co-receptors found on mature T cells. At this stage, upregulation of RAG1/RAG2 recombinases induces VDJ rearrangement of the β-chain and γδ- loci of the TCR. Meanwhile, TdTs (Terminal Deoxynucleotidyl Transferases) add N- and P- nucleotides to the end of the V, D and J segments to diversify the specificity of the newly forming T cell receptor. Determination of the αβ/γδ lineage fate depends on TCR signal strength. This model of lineage determination predicts that the γδ TCR transduces a stronger signal directing cells to the γδ lineage, while a pre-TCR transduces a weaker signal directing progenitors to the αβ lineage (25, 28).
DN2 thymocytes are differentiated from DN1 progenitors. These progenitors were named double negative as they do not express the CD4 or CD8 co-receptors found on mature T cells. At this stage, upregulation of RAG1/RAG2 recombinases induces VDJ rearrangement of the β-chain and γδ- loci of the TCR. Meanwhile, TdTs (Terminal Deoxynucleotidyl Transferases) add N- and P- nucleotides to the end of the V, D and J segments to diversify the specificity of the newly forming T cell receptor. Determination of the αβ/γδ lineage fate depends on TCR signal strength. This model of lineage determination predicts that the γδ TCR transduces a stronger signal directing cells to the γδ lineage, while a pre-TCR transduces a weaker signal directing progenitors to the αβ lineage (25, 28).
 ISP thymocytes form an intermediate stage between DN4s and DPs. During this stage, the TCRα-chain undergoes rearrangement to form the αβ-TCR before transitioning to the DP stage.
ISP thymocytes form an intermediate stage between DN4s and DPs. During this stage, the TCRα-chain undergoes rearrangement to form the αβ-TCR before transitioning to the DP stage. DP thymocytes differentiate from the ISP stage and can be distinguished by the expression of both CD4 and CD8 co-receptors. The fate of DP cells depends on the interaction of their αβ-TCR with MHCI/II molecules expressed on cortical epithelial cells. Upon TCR interaction with self pMHC molecules, there are 3 possible outcomes:
DP thymocytes differentiate from the ISP stage and can be distinguished by the expression of both CD4 and CD8 co-receptors. The fate of DP cells depends on the interaction of their αβ-TCR with MHCI/II molecules expressed on cortical epithelial cells. Upon TCR interaction with self pMHC molecules, there are 3 possible outcomes: CD8αα IELs differentiate as an alternative pathway to clonal deletion of autoreactive T cells. Autoreactive DP cells which do not receive a costimulation signal through B7/CD28 escape clonal deletion during the process of negative selection in the thymic medulla. These cells downregulate both CD4 and CD8 co-receptors to become TCRαβ+ DN cells. From here, they migrate to gut tissues where they differentiate to CD8αα IELs and function to regulate intestinal homeostasis and maintain epithelial barrier function (27).
CD8αα IELs differentiate as an alternative pathway to clonal deletion of autoreactive T cells. Autoreactive DP cells which do not receive a costimulation signal through B7/CD28 escape clonal deletion during the process of negative selection in the thymic medulla. These cells downregulate both CD4 and CD8 co-receptors to become TCRαβ+ DN cells. From here, they migrate to gut tissues where they differentiate to CD8αα IELs and function to regulate intestinal homeostasis and maintain epithelial barrier function (27). γδ-T cells diverge from αβ-T cells at the DN2 progenitor stage. γδ-T cells represent a small subset of T cells found in tissues of both lymphoid and non-lymphoid origin. Unlike the αβ-TCR, the γδ-TCR recognizes lipid antigens and doesn’t require antigen presentation by MHC molecules.
γδ-T cells diverge from αβ-T cells at the DN2 progenitor stage. γδ-T cells represent a small subset of T cells found in tissues of both lymphoid and non-lymphoid origin. Unlike the αβ-TCR, the γδ-TCR recognizes lipid antigens and doesn’t require antigen presentation by MHC molecules. CD4+CD8low cells differentiate from DP thymocytes through positive selection. CD4+CD8low thymocytes pass lineage commitment and differentiate into CD8+ and CD4+ T cells. According to the kinetic signaling model, the lineage fate decision depends on the persistence of TCR signaling in CD4+CD8low cells. Persistence of TCR signaling in CD4+CD8low intermediate thymocytes block IL-7 signaling and induces differentiation into CD4+ T cells. Cessation or disruption of TCR signaling permits IL-7 signaling resulting in co-receptor reversal and differentiation to CD8+ cells (28).
CD4+CD8low cells differentiate from DP thymocytes through positive selection. CD4+CD8low thymocytes pass lineage commitment and differentiate into CD8+ and CD4+ T cells. According to the kinetic signaling model, the lineage fate decision depends on the persistence of TCR signaling in CD4+CD8low cells. Persistence of TCR signaling in CD4+CD8low intermediate thymocytes block IL-7 signaling and induces differentiation into CD4+ T cells. Cessation or disruption of TCR signaling permits IL-7 signaling resulting in co-receptor reversal and differentiation to CD8+ cells (28). CD8+ T cells or cytotoxic T cells differentiate from CD4+CD8low progenitors and recognize antigens presented on MHC class I molecules. Mature CD8+ T cells which are ready to egress the thymus can be identified by downregulation of CD24 and overexpression of S1PR.
CD8+ T cells or cytotoxic T cells differentiate from CD4+CD8low progenitors and recognize antigens presented on MHC class I molecules. Mature CD8+ T cells which are ready to egress the thymus can be identified by downregulation of CD24 and overexpression of S1PR. CD4+ T cells or T helper cells differentiate from the CD4+CD8low progenitors and recognize antigens presented on MHC class II molecules. Mature CD4+ T cells which are ready to egress the thymus can be identified by downregulation of CD24 and overexpression of S1PR. In the periphery, mature CD4+ T cells differentiate into different T helper subsets upon encountering an antigen.
CD4+ T cells or T helper cells differentiate from the CD4+CD8low progenitors and recognize antigens presented on MHC class II molecules. Mature CD4+ T cells which are ready to egress the thymus can be identified by downregulation of CD24 and overexpression of S1PR. In the periphery, mature CD4+ T cells differentiate into different T helper subsets upon encountering an antigen. T regulatory cells are specialized T cells responsible for protecting the organism from autoimmune responses by regulating CD4+ and CD8+ T cell cytotoxic activity. One of the transcription factors important for Treg cell differentiation is FOXP3. Mice with the “Scurfy” mutation of the FOXP3 gene leading to ablation of FOXP3 expression exhibit multi-system autoimmune disorders.
T regulatory cells are specialized T cells responsible for protecting the organism from autoimmune responses by regulating CD4+ and CD8+ T cell cytotoxic activity. One of the transcription factors important for Treg cell differentiation is FOXP3. Mice with the “Scurfy” mutation of the FOXP3 gene leading to ablation of FOXP3 expression exhibit multi-system autoimmune disorders. DP thymocytes that express a rearranged Va14i-TCR interact with glycolipids presented on CD1d molecules and differentiate into NKT cells (23).
DP thymocytes that express a rearranged Va14i-TCR interact with glycolipids presented on CD1d molecules and differentiate into NKT cells (23). ETP/DN1s are one of the first T cell progenitor populations that have been identified in the thymus. These progenitors are named double negative as they do not express the CD4 or CD8 co-receptors found on mature T cells. Additionally, they do not express the CD3/TCR complex.
ETP/DN1s are one of the first T cell progenitor populations that have been identified in the thymus. These progenitors are named double negative as they do not express the CD4 or CD8 co-receptors found on mature T cells. Additionally, they do not express the CD3/TCR complex.


Follow Us